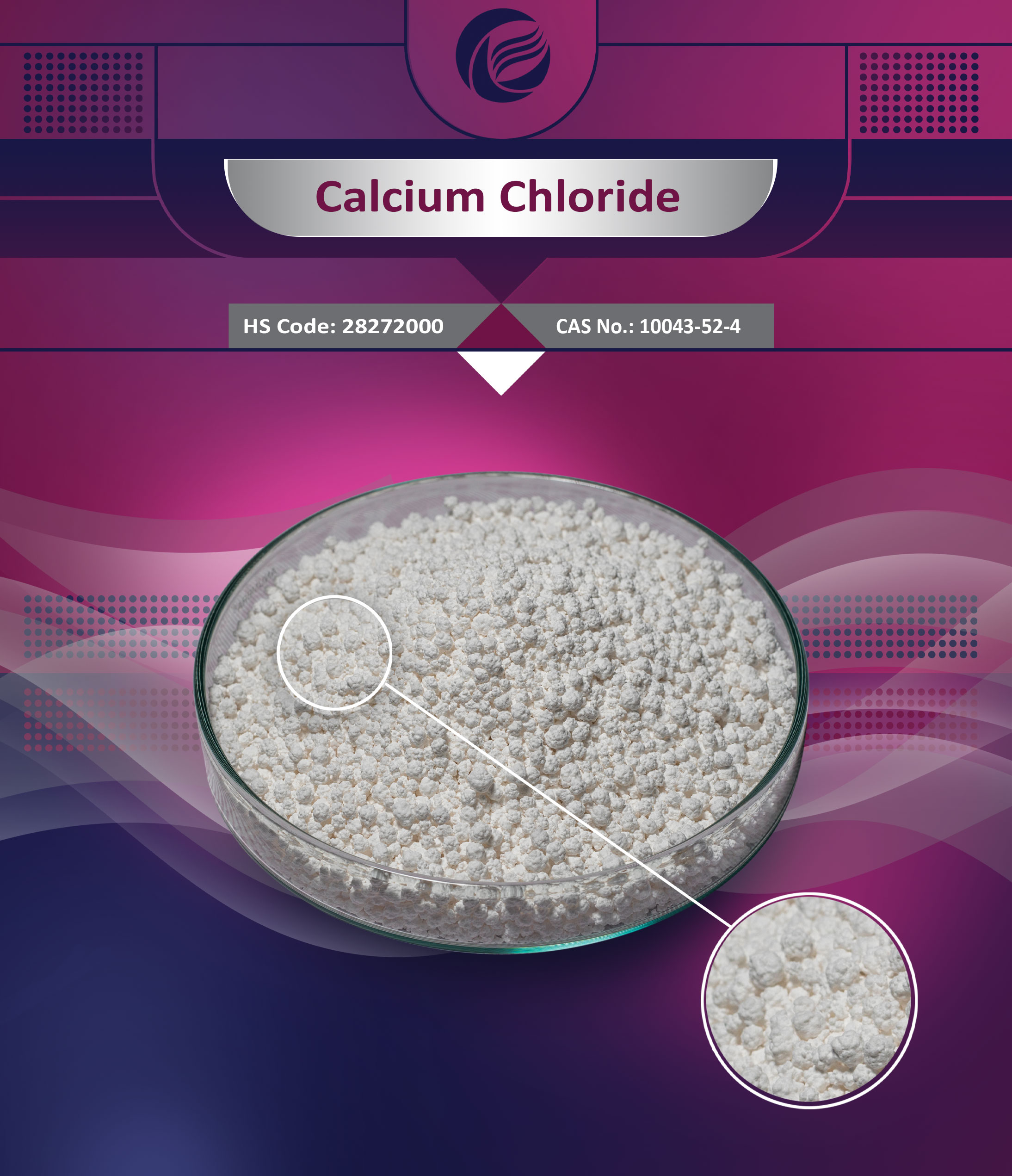
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its … Calcium chloride is an inorganic compound, a salt with the chemical formula CaCl2. It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide. What is calcium and what does it do? Calcium is a mineral your body needs to build and maintain strong bones and to carry out many important functions. Calcium is the most abundant mineral … · Calcium is an essential mineral that’s key to your bones and overall health. It’s recommended that most adults consume at least 1,000 mg of calcium a day. 4 days ago · Calcium, one of the alkaline earth metals, chemical symbol Ca, atomic number 20, the most abundant metallic element in the human body. Learn more about Calcium uses, effectiveness, possible side effects, interactions, dosage, user ratings and products that contain Calcium. When the body has enough calcium, a different hormone called calcitonin works to do the opposite: it lowers calcium levels in the blood by stopping the release of calcium from bones … · Your body needs calcium for many reasons. Learn what foods are high in calcium and how much calcium you need in a healthy diet. · Calcium is a mineral that's essential for bone health, found in yogurt, milk, spinach, and salmon. Supplements can lower the risk of fractures. · People need calcium for bone health and other functions. Find out why people need calcium, which foods provide it, and what happens if they consume too little. Calcium is one of the body's electrolytes, which are minerals that carry an electric charge when dissolved in body fluids such as blood, but most of the body's calcium is uncharged. One use for calcium is as a desiccant.Anything that qualifies as a desiccant means it’s used as a drying agent. Using calcium chloride in foods helps to absorb any moisture that would createan environment where bacteria would thrive. It is also used both pre- and post-harvest to maintain firmness, reduce decay, and prevent certain diseases in fruit...See full list on ingredi.comThe uses of calcium chloride are plentiful, which leads tothe question on whether it’s safe to consume. The use of calcium chloride infood relate to the fact that it is extremely salty in taste. It is used inbrines, giving a salty taste without adding in all the sodium. Using calcium chloride in food serves as a firming agent aswell, and it is comm...See full list on ingredi.comAs far as food additives go, calciumchloride provides plenty of benefits to your health! This is especially true when it’s used in sports drinks. It acts as one of the electrolytes, helping your body maintain fluid through activities and helps you to maintain proper muscle and nerve functioning. When calciumchloride is used in sports drinks, it a...See full list on ingredi.comCalciumchloride is an ionic compound of calcium and chlorine. It is highly soluble in water and it is deliquescent. It is a salt that is solid at room temperature, and it behaves as a typical ionic halide. It has several common applications such as brine for refrigeration plants, ice and dust control on roads, and in cement. Easy-to-read patient leaflet for CalciumChloride. Includes indications, proper use, special instructions, precautions, and possible side effects. · Scientifically represented as CaCl₂, Calcium Chloride is a white crystalline salt composed of calcium and chlorine. It plays a crucial role across numerous industries, ranging from food processing and water treatment to construction and agriculture. 1 day ago · Calciumchloride (CaCl2) is an ionic compound, a salt of calcium and chlorine, that exists as a white crystalline solid at room temperature. This compound is highly soluble in water and is notable for its pronounced hygroscopic property, meaning it readily attracts and holds water molecules from the surrounding air. Is calcium chloride a good supplement?Calcium chloride has a very salty taste and can cause mouth and throat irritation at high concentrations, so it is typically not the first choice for long-term oral supplementation (as a calcium supplement).What is calcium?Calcium, with the chemical symbol Ca, is a chemical element with an atomic number of 20 and a molar mass of 40.08 grams. Its physical properties include a melting point of 1115°K or 842 °C (1548 °F) and a boiling point of 1757 or 1484 °C (2703 °F). Calcium has a density of 1550 kg/m3 and a molar volume of 26.20 cm3.Is calcium chloride included in this list?Calcium chloride is included on this list. Limit: When ready for use, the end-use concentration is not to exceed 17 ppm. 40 CFR 180.940 (b) (USEPA); U.S. National Archives and Records Administration's Electronic Code of Federal Regulations. · Calcium chloride (CaCl₂) is a white, crystalline salt composed of calcium and chlorine. It’s highly soluble in water, making it extremely versatile. It can be naturally derived from limestone or created synthetically as a byproduct of other chemical processes like the Solvay process. Calciumchloride is an ionic compound of calcium and chlorine. It is highly soluble in water and it is deliquescent. It is a salt that is solid at room temperature, and it behaves as a typical ionic halide. It has several common applications such as brine for refrigeration plants, ice and dust control on roads, and in cement. Easy-to-read patient leaflet for CalciumChloride. Includes indications, proper use, special instructions, precautions, and possible side effects. · Scientifically represented as CaCl₂, Calcium Chloride is a white crystalline salt composed of calcium and chlorine. It plays a crucial role across numerous industries, ranging from food processing and water treatment to construction and agriculture. 1 day ago · Calciumchloride (CaCl2) is an ionic compound, a salt of calcium and chlorine, that exists as a white crystalline solid at room temperature. This compound is highly soluble in water and is notable for its pronounced hygroscopic property, meaning it readily attracts and holds water molecules from the surrounding air. · Calcium chloride (CaCl₂) is a white, crystalline salt composed of calcium and chlorine. It’s highly soluble in water, making it extremely versatile. It can be naturally derived from limestone or created synthetically as a byproduct of other chemical processes like the Solvay process.